
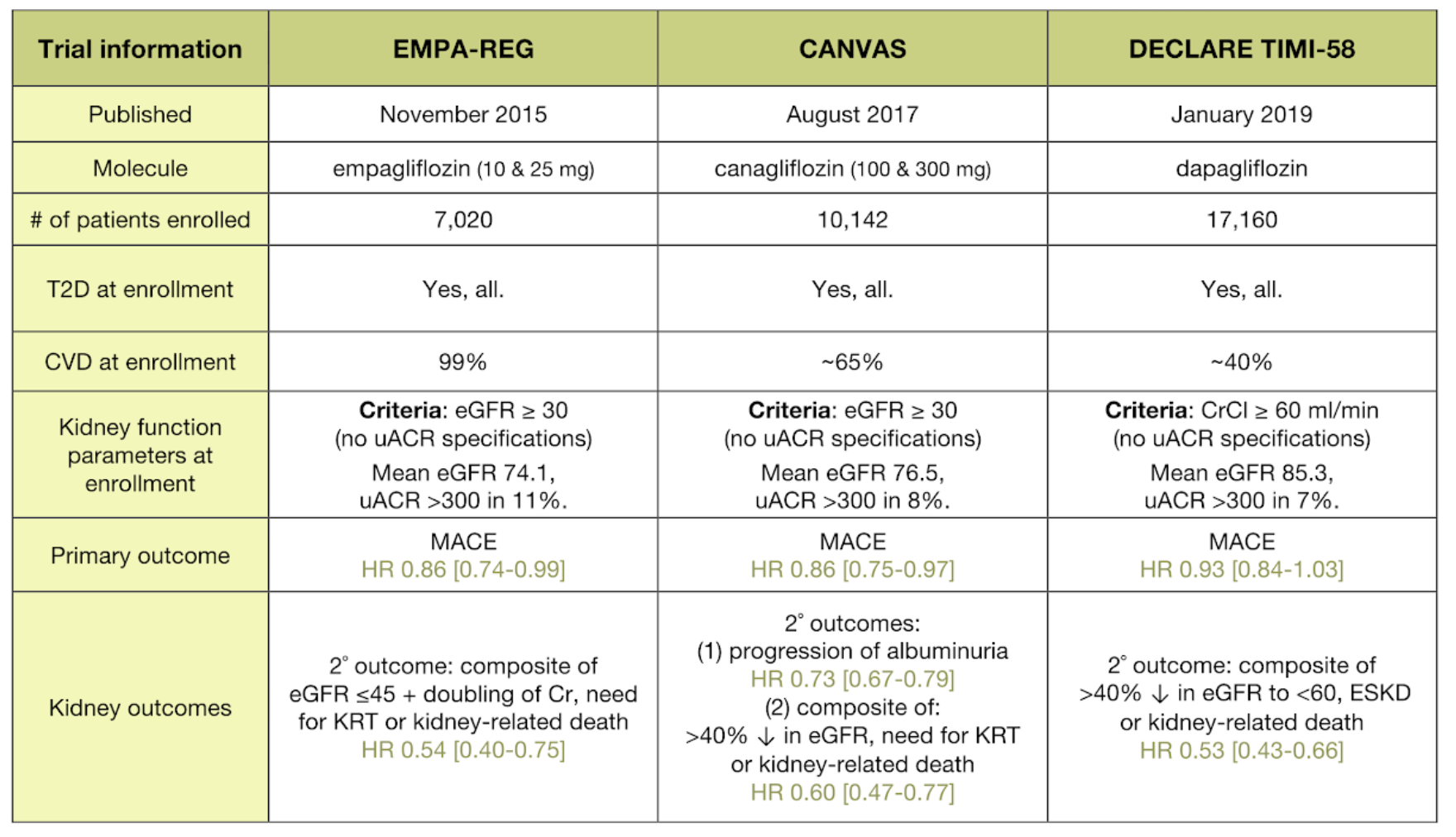
DECLARE-TIMI 58 is the largest study to address this question with an SGLT-2 inhibitor in patients with T2DM and with established CV disease and without CV disease but with multiple risk factors. Conclusion: The DECLARETIMI 58 trial is testing the hypotheses that dapagliflozin is safe (does not increase) and may reduce the occurrence of major CV. 0231.ConclusionThe DECLARE-TIMI 58 trial is testing the hypotheses that dapagliflozin is safe (does not increase) and may reduce the occurrence of major CV events. This event-driven trial will continue until at least 1,390 subjects have a MACE outcome, thereby providing >99% power to test for the primary outcome of safety of dapagliflozin measured by rejecting the hypothesis that the upper bound of the CI >1.3 for the primary outcome of MACE, as well as 85% power to detect a 15% relative risk reduction in MACE and an estimated 87% power to detect a 20% reduction in the composite of CV death or hospitalization for heart failure at a 1-sided α level of. In the safety analysis of the DECLARE-TIMI 58 trial, genital infections were more common in the dapagliflozin group compared with placebo (74 versus 4. The co-primary efficacy outcomes are the composite of CV death, myocardial infarction, or ischemic stroke and the composite of CV death or hospitalization for heart failure. The primary safety outcome is the time to the first event of the composite of CV death, myocardial infarction, or ischemic stroke (major adverse cardiovascular events MACEs). Patients were randomized in a 1:1 fashion to dapagliflozin 10 mg or matching placebo. We randomly assigned 6263 patients with heart failure and a left ventricular ejection fraction of more than 40 to receive dapagliflozin (at a dose of 10 mg once daily) or matching placebo, in. Effect of Dapagliflozin on Atrial Fibrillation in Patients With Type 2 Diabetes Mellitus: Insights From the DECLARE-TIMI 58 Trial. People with type 2 diabetes and either risk factors for or established atherosclerotic cardiovascular disease were randomly assigned (1:1) to receive oral dapagliflozin 10 mg or placebo once daily. Previous trials of SGLT-2 inhibitors showed reductions in cardiovascular (CV) events, including CV death and hospitalization for heart failure, and ischemic events in patients with atherosclerotic cardiovascular disease (ASCVD).Research design and methodsDECLARE-TIMI 58 (NCT01730534) is a phase 3b randomized, double-blind, placebo-controlled trial designed to evaluate the CV safety and efficacy of dapagliflozin that has completed randomization of 17,160 patients with T2DM and a history of either established ASCVD (n=6,971) or multiple risk factors for ASCVD (n=10,189). The DECLARE-TIMI 58 trial was a double-blind, multicentre, randomised, placebo-controlled study.

In addition to improving blood glucose control, treatment with dapagliflozin results in glucose-induced osmotic diuresis, weight loss, and blood pressure lowering. BackgroundDapagliflozin is a sodium-glucose co-transporter-2 (SGLT-2) inhibitor that reduces blood glucose in patients with type 2 diabetes mellitus (T2DM) by promoting glycosuria via inhibiting urinary glucose reabsorption.


 0 kommentar(er)
0 kommentar(er)
